
Clinical Trial Software Market Surges to US$ 2.3 Billion by 2031, Propelled by 14.3% CAGR, States CoherentMI
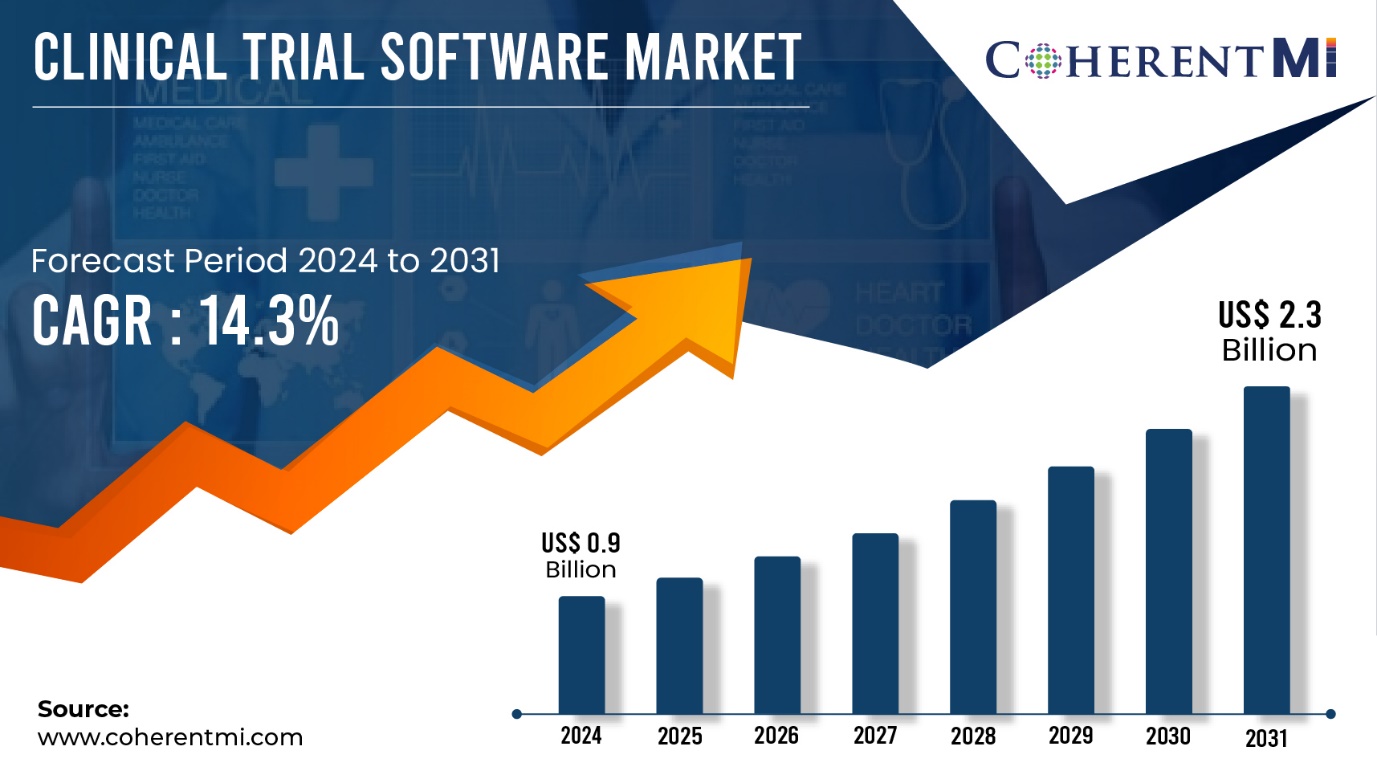
The Clinical Trial Software Market is estimated to be valued at US$ 0.9 Bn in 2024 and is expected to exhibit a CAGR of 14.3% over the forecast period 2024-2031, as highlighted in a new report published by CoherentMI. Top Players covered Advarra, Arisglobal, AssistRx, Calyx, Clario and Among Others.
/EIN News/ -- Burlingame, Sept. 10, 2024 (GLOBE NEWSWIRE) -- CoherentMI published a report, titled, Clinical Trial Software Market is estimated to value at US$ 0.9 Billion in the year 2024, and is anticipated to reach US$ 2.3 Billion by 2031, with growing at a CAGR of 14.3% during forecast period 2024-2031. The biopharmaceutical industry has been steadily increasing investment in R&D year after year to develop new drugs and treatments. As complex clinical trials are an important part of drug development, companies are allocating greater budgets to clinical trial processes and management. Furthermore, The COVID-19 pandemic accelerated the adoption of digital health solutions as social distancing norms encouraged remote monitoring of patients. Telehealth emerged as a viable method to screen, diagnose and treat patients without physical visits. This highlighted how modern technologies can simplify clinical trials while maintaining participant safety.
Market Report Scope:
| Report Coverage | Details |
| Market Revenue in 2024: | US$ 0.9 Billion |
| Estimated Value by 2031: | US$ 2.3 Billion |
| Growth Rate: | Poised to grow at a CAGR of 14.3% |
| Historical Data: | 2019–2023 |
| Forecast Period: | 2024–2031 |
| Forecast Units: | Value (USD Million/Billion) |
| Report Coverage: | Revenue Forecast, Competitive Landscape, Growth Factors, and Trends |
| Segments Covered: | By Features of Software, By Type of Deployment |
| Geographies Covered: | Global |
| Major Players: | Advarra, Arisglobal, AssistRx, Calyx, Clario and Among Others. |
| Growth Drivers: | • Increased digitalization in clinical trials |
| • Rising demand for patient-centric clinical trial solutions | |
| Restraints & Challenges: | • High costs associated with implementation |
Market Dynamics:
The clinical trial software market is driven by the increasing digitization in the healthcare sector and rising adoption of clinical trial management systems. Clinical trial software enables efficient management of complex clinical trial processes by streamlining workflows, improving communication among research sites and sponsors, and ensuring compliance. This enhances the productivity and speed of clinical trials.
Key Market Takeaways:
The global clinical trial software market size is anticipated to witness a CAGR of 14.3% during the forecast period 2024-2031, owing to increasing digitalization and growing R&D investments in pharmaceutical and biotech industries. On the basis of features of software, EDC segment is expected to hold a dominant position, owing to its widespread adoption for simplifying clinical trial data management. On the basis of type of deployment, on-cloud segment is expected to hold a dominant position over the forecast period, due to benefits like seamless collaboration, remote monitoring and automatic updates.
On the basis of region, North America is expected to hold a dominant position over the forecast period, due to presence of majority global players, fast adoption of advanced technologies and high clinical research expenditure in the region. Key players operating in the clinical trial software market include Advarra, Arisglobal, AssistRx, Calyx, Clario, IBM, IQVIA, Medidata, Oracle, Signant Health, and Veeva. These players are focusing on new product launches and partnerships with pharmaceutical companies to strengthen their market position and capture higher shares in the overall clinical trial software market.
Market Trends:
Cloud-based clinical trial software is gaining popularity in the market. Cloud deployment reduces IT costs and provides remote access along with centralized data management. It helps clinical trial managers in making real-time decisions and resolving issues quickly. Another key trend is the advent of artificial intelligence in clinical trials. AI assists in patient recruitment, monitoring, and analysis of huge clinical data to gain meaningful insights for researchers. This accelerates various clinical processes and results.
Electronic Data Capture (EDC): The EDC feature allows monitoring and documentation of clinical trial data in electronic format replacing traditional paper-based methods. EDC software helps in efficiently planning, collecting, validating and managing clinical trial data in real-time. Streamlining data collection and management reduces costs and speeds up the overall clinical trial process. EDC segment is expected to hold the major share of over 35% of the overall clinical trial software market during the forecast period owing to its widespread adoption for simplifying data handling.
Cloud and On-premises Deployment: Clinical trial software can be deployed either through cloud or on-premises model. The cloud-based deployment or Software as a Service (SaaS) model is gaining popularity as it eliminates upfront capital expenses and provides flexibility to access software from any location. The on-cloud segment is anticipated to hold over 55% share of the total market size by 2031, attributed to benefits like seamless collaboration, remote monitoring and automatic updates. However, the on-premises deployment continues to find applications where data privacy and regulatory concerns necessitate local data storage.
Recent Development:
- In August 2023, Texas Tech University Health Sciences Center collaborated with Deep 6 AI to launch an AI program for clinical trials.
- In August 2023, Globant partnered with Medocity to accelerate digitalization in clinical research.
Get a detailed analysis on regions, market segments, and companies: https://www.coherentmi.com/industry-reports/clinical-trial-software-market
Clinical Trial Software Market Segmentation:
-
By Features of Software -
- EDC
- eCOA/ePRO
- eConsent
-
By Type of Deployment -
- On-cloud
- On-premises
The research provides answers to the following key questions:
- What is the estimated growth rate of the market for the forecast period 2024-2031?
- What will be the market size during the estimated period?
- What are the key driving forces responsible for shaping the fate of the Clinical Trial Software market during the forecast period?
- Who are the major market vendors and what are the winning strategies that have helped them occupy a strong foothold in the Clinical Trial Software market?
- What are the prominent market trends influencing the development of the Clinical Trial Software market across different regions?
- What are the major threats and challenges likely to act as a barrier in the growth of the Clinical Trial Software market?
- What are the major opportunities the market leaders can rely on to gain success and profitability?
Purchase Latest Edition of this Research Report @ https://www.coherentmi.com/industry-reports/clinical-trial-software-market/buynow
Key insights provided by the report that could help you take critical strategic decisions?
- Regional report analysis highlighting the consumption of products/services in a region also shows the factors that influence the market in each region.
- Reports provide opportunities and threats faced by suppliers in the Clinical Trial Software industry around the world.
- The report shows regions and sectors with the fastest growth potential.
- A competitive environment that includes market rankings of major companies, along with new product launches, partnerships, business expansions, and acquisitions.
- The report provides an extensive corporate profile consisting of company overviews, company insights, product benchmarks, and SWOT analysis for key market participants.
- This report provides the industry's current and future market outlook on the recent development, growth opportunities, drivers, challenges, and two regional constraints emerging in advanced regions.
Browse Related Reports:
At Home Testing Kits Market: The at home testing kits market is estimated to be valued at USD 20.40 Bn in 2024 and is expected to reach USD 31.15 Bn by 2031, growing at a compound annual growth rate (CAGR) of 6.2% from 2024 to 2031.
Clinical Trial Patient Recruitment Services Market: The clinical trial patient recruitment services market is estimated to be valued at USD 9.4 Bn in 2024 and is expected to reach USD 19.1 Bn by 2031, growing at a compound annual growth rate (CAGR) of 8.2% from 2024 to 2031.
Australia Blood Ketone Meter Market: The Australia Blood Ketone Meter Market is estimated to be valued at USD 10.9 Mn in 2024 and is expected to reach USD 18.8 Mn by 2031, growing at a compound annual growth rate (CAGR) of 7% from 2024 to 2031.
U.S. Colorectal Cancer Screening Market: U.S. colorectal cancer screening market size was valued at US$ 5.95 Bn in 2023 and is expected to reach US$ 10.62 Bn by 2031, grow at a compound annual growth rate (CAGR) of 7.5% from 2024 to 2031.
Author of this marketing PR:
Ravina Pandya, PR Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. With an MBA in E-commerce, she has an expertise in SEO-optimized content that resonates with industry professionals.
About Us:
At CoherentMI, we are a leading global market intelligence company dedicated to providing comprehensive insights, analysis, and strategic solutions to empower businesses and organizations worldwide. Moreover, CoherentMI is a subsidiary of Coherent Market Insights Pvt Ltd., which is a market intelligence and consulting organization that helps businesses in critical business decisions. With our cutting-edge technology and experienced team of industry experts, we deliver actionable intelligence that helps our clients make informed decisions and stay ahead in today's rapidly changing business landscape.

Mr. Shah
CoherentMI,
U.S.: +1-650-918-5898
U.K: +44-020-8133-4027
Australia: +61-2-4786-0457
INDIA: +91-848-285-0837
Email: sales@coherentmi.com
Website: https://www.coherentmi.com
Distribution channels: Banking, Finance & Investment Industry, Business & Economy, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release


